It’s simple and secure, so you can focus on innovation.
SafeQMS is a cloud-based document control system specially designed for the needs of medical tech startups and emerging companies.
Will your quality system pass?
As a medical device manufacturer, building a quality system, which is compliant with FDA requirements and easy to operate is a challenge. Compliance with ISO 13845 or other standards adds another layer of complexity. SafeQMS makes operations that follow your quality system easier and facilitates audits.
Beyond the actual SOP's informed, we provide the support and training to ensure that you are ready for any regulatory body inspection.

Manage the complexity of your quality system with ease.
SafeQMS gives you and your employees a current, accurate view of your quality system wherever you have an internet connection.
Give access to those who need it, and restrict access to those who don’t. Manage document signings 100% digitally. Automatically maintain a record of how documents change over time.

SafeQMS helps your medical miracle reach patients.
SafeQMS was built with the input of ISO auditors with decades of experience working with and auditing quality systems. It's designed specifically for the needs of smaller and emerging companies.
It has everything you need to manage your quality system effectively, without the feature bloat of a system designed for large organizations.
It’s priced with you in mind: buy the features you need, and add more modules in the future as your company grows.

Features
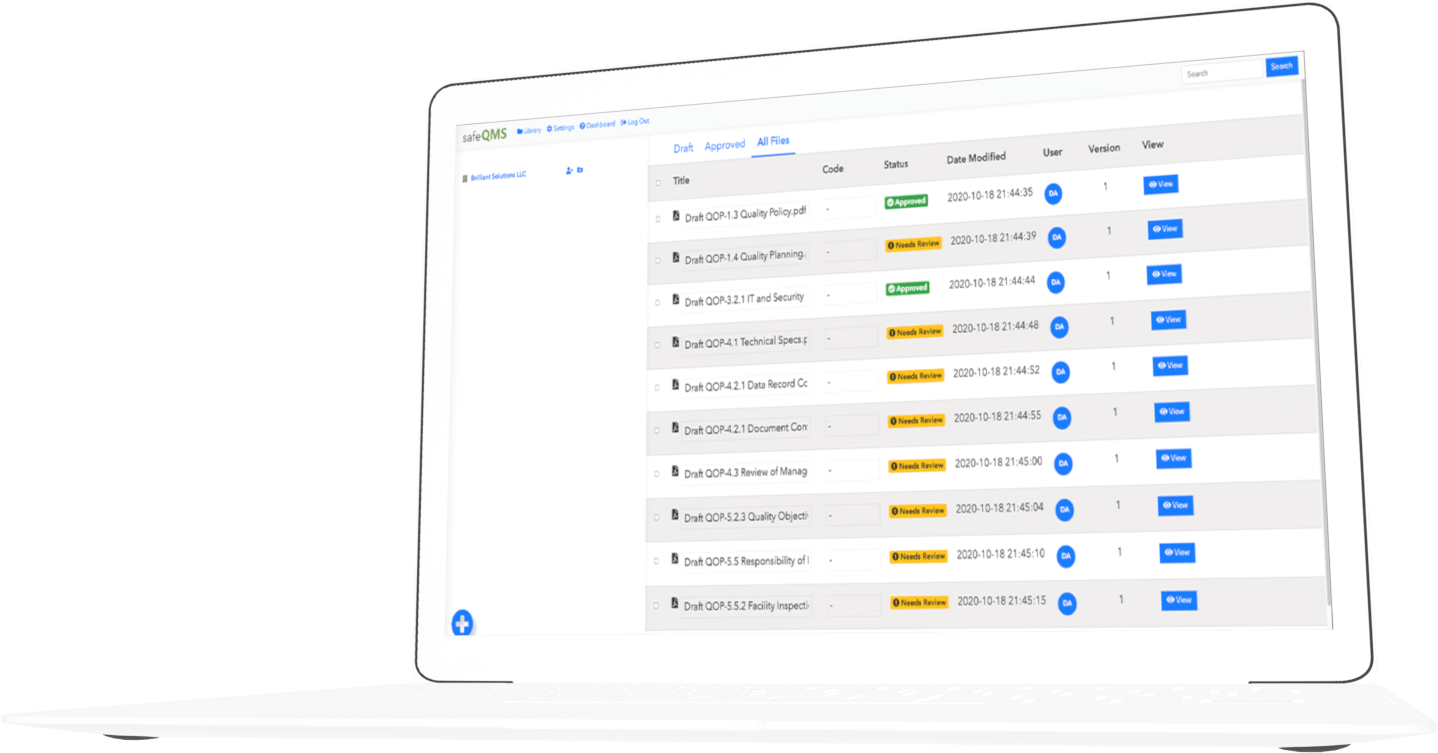
Track your documents over time
SafeQMS lets you track revision history, compare differences between versions, and produce audit trails.
Control access to critical info
Control who in your organization can access each document.
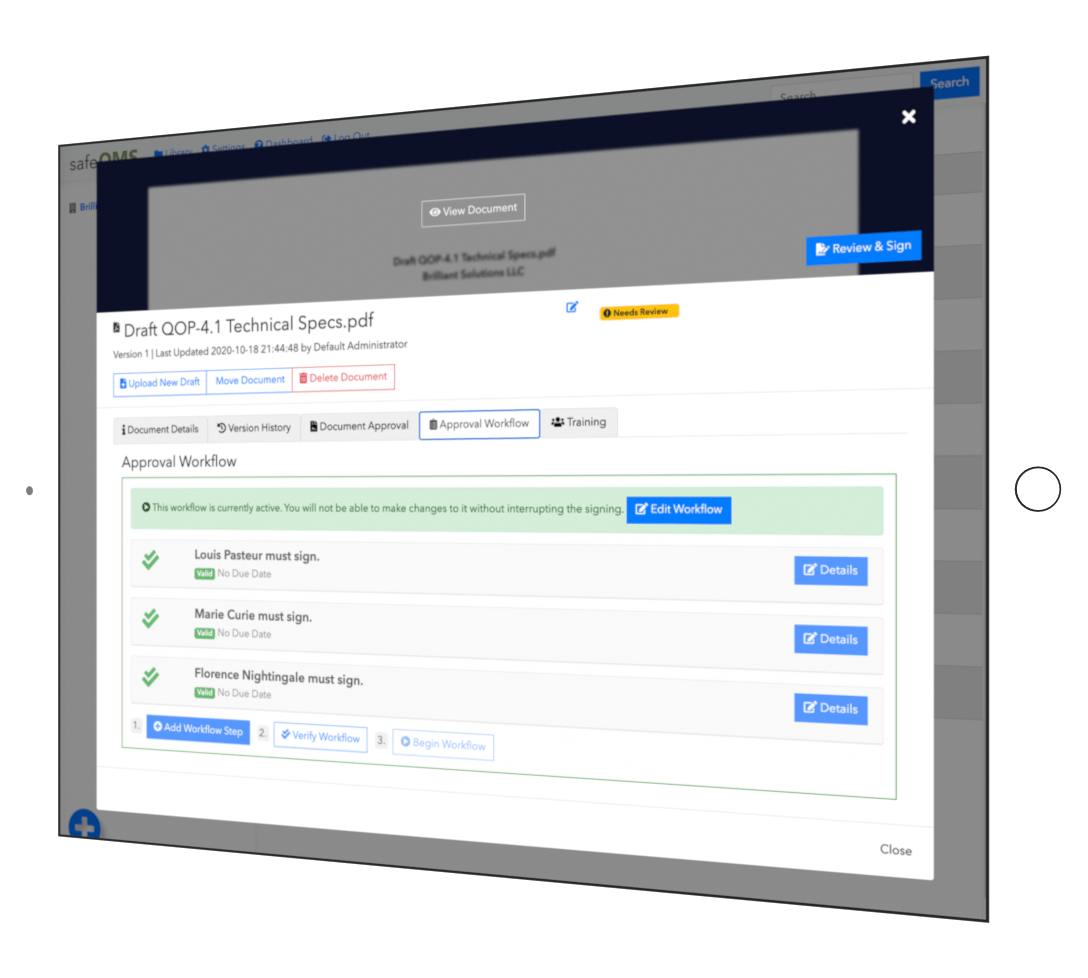
Manage document approval
Construct workflows for people and groups in your company to review new or revised SOPs and forms.
21 CFR Compliant
SafeQMS is 21 CFR part 11 validated and compliant. Among our security methods, we encrypt data at rest, enforce strong passwords and use 2 factor authentication.
Assign users to train
Assign users to train on documents of your quality system.
Email Alerts
Receive email alerts when new documents are added, when it’s your time to sign, and more.
Why Choose SafeQMS?
It’s 21 CFR Part 11 compliant.
It’s built with the insight of people who have decades of experiences in med-tech.
It’s designed with you in mind, so your product can reach patients and you can focus on growing your business.
Contact Us
We would enjoy showing you a demo of SafeQMS and discussing integrating it into your workflow.
